Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The chemical symbol for Nitrogen is N. Atomic Mass of Nitrogen Atomic mass of Nitrogen is 14.0067 u. The sum of the atomic number Z and the number of neutrons N gives the mass number A of an atom. Since protons and neutrons have approximately the same mass (and the mass of the electrons is negligible for many purposes) and the mass defect of nucleon binding is always small compared to the nucleon mass, the atomic mass of any atom, when expressed in unified atomic mass units (making a quantity. Atomic Number of Nitrogen Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The chemical symbol for Nitrogen is N. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.
Nitrogen is present in almost all proteins and plays important roles in both biochemical applications and industrial applications. Nitrogen forms strong bonds because of its ability to form a triple bond with its self, and other elements. Thus, there is a lot of energy in the compounds of nitrogen. Before 100 years ago, little was known about nitrogen. Now, nitrogen is commonly used to preserve food, and as a fertilizer.
Introduction
Nitrogen is found to have either 3 or 5 valence electrons and lies at the top of Group 15 on the periodic table. It can have either 3 or 5 valence electrons because it can bond in the outer 2p and 2s orbitals. Molecular nitrogen ((N_2)) is not reactive at standard temperature and pressure and is a colorless and odorless gas.
Nitrogen is a non-metal element that occurs most abundantly in the atmosphere, nitrogen gas (N2) comprises 78.1% of the volume of the Earth’s air. It only appears in 0.002% of the earth's crust by mass. Compounds of nitrogen are found in foods, explosives, poisons, and fertilizers. Nitrogen makes up DNA in the form of nitrogenous bases as well as in neurotransmitters. It is one of the largest industrial gases, and is produced commercially as a gas and a liquid.
| Name and Symbol | Nitrogen, N |
| Category | non-metal |
| Atomic Weight | 14.0067 |
| Group | 15 |
| Electron Configuration | 1s2 2s2 2p3 |
| Valence Electrons | 2, 5 |
| Phase | Gas |
History
Nitrogen, which makes up about 78% of our atmosphere, is a colorless, odorless, tasteless and chemically unreactive gas at room temperature. It is named from the Greek nitron + genes for soda forming. For many years during the 1500's and 1600's scientists hinted that there was another gas in the atmosphere besides carbon dioxide and oxygen. It was not until the 1700's that scientists could prove there was in fact another gas that took up mass in the atmosphere of the Earth.
Discovered in 1772 by Daniel Rutherford (and independently by others such as Priestly and Cavendish) who was able to remove oxygen and carbon dioxide from a contained tube full of air. He showed that there was residual gas that did not support combustion like oxygen or carbon dioxide. While his experiment was the one that proved that nitrogen existed, other experiments were also going in London where they called the substance 'burnt' or 'dephlogisticated air'.
Nitrogen is the fourth most abundant element in humans and it is more abundant in the known universe than carbon or silicon. Most commercially produced nitrogen gas is recovered from liquefied air. Of that amount, the majority is used to manufacture ammonia ((NH_3)) via the Haber process. Much is also converted to nitric acid ((HNO_3)).
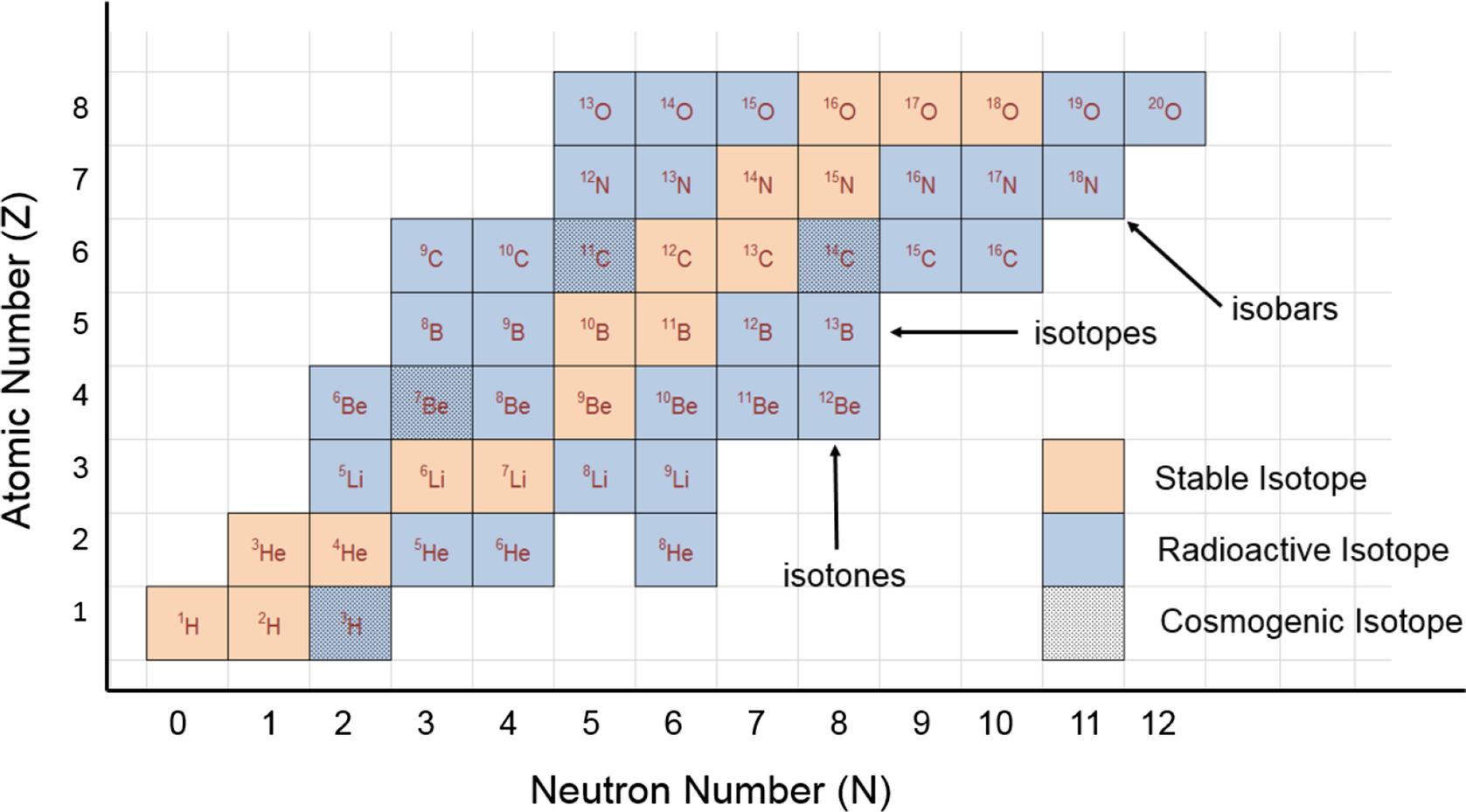
Isotopes
Nitrogen has two naturally occurring isotopes, nitrogen-14 and nitrogen-15, which can be separated with chemical exchanges or thermal diffusion. Nitrogen also has isotopes with 12, 13, 16, 17 masses, but they are radioactive.

- Nitrogen 14 is the most abundant form of nitrogen and makes up more than 99% of all nitrogen found on Earth. It is a stable compound and is non-radioactive. Nitrogen-14 has the most practical uses, and is found in agricultural practices, food preservation, biochemicals, and biomedical research. Nitrogen-14 is found in abundance in the atmosphere and among many living organisms. It has 5 valence electrons and is not a good electrical conductor.
- Nitrogen-15 is the other stable form of nitrogen. It is often used in medical research and preservation. The element is non-radioactive and therefore can also be sometimes used in agricultural practices. Nitrogen-15 is also used in brain research, specifically nuclear magnetic resonance spectroscopy (NMR), because unlike nitrogen-14 (nuclear spin of 1), it has a nuclear spin of 1/2 which has benefits when it comes to observing MRI research and NMR observations. Lastly, nitrogen-15 can be used as label or in some proteins in biology. Scientists mainly use this compound for research purposes and have not yet seen its full potential for uses in brain research.
Compounds
The two most common compounds of nitrogen are Potassium Nitrate (KNO3) and Sodium Nitrate (NaNO3). These two compounds are formed by decomposing organic matter that has potassium or sodium present and are often found in fertilizers and byproducts of industrial waste. Most nitrogen compounds have a positive Gibbs free energy (i.e., reactions are not spontaneous).
The dinitrogen molecule ((N_2)) is an 'unusually stable' compound, particularly because nitrogen forms a triple bond with itself. This triple bond is difficult hard to break. For dinitrogen to follow the octet rule, it must have a triple bond. Nitrogen has a total of 5 valence electrons, so doubling that, we would have a total of 10 valence electrons with two nitrogen atoms. The octet requires an atom to have 8 total electrons in order to have a full valence shell, therefore it needs to have a triple bond. The compound is also very inert, since it has a triple bond. Triple bonds are very hard to break, so they keep their full valence shell instead of reacting with other compounds or atoms. Think of it this way, each triple bond is like a rubber band, with three rubber bands, the nitrogen atoms are very attracted to each other.

Nitrides
Nitrides are compounds of nitrogen with a less electronegative atom; in other words it's a compound with atoms that have a less full valence shell. These compounds form with lithium and Group 2 metals. Nitrides usually has an oxidation state of -3.
[3Mg + N_2 rightarrow Mg_3N_2 label{1}]
When mixed with water, nitrogen will form ammonia and, this nitride ion acts as a very strong base.
[N + 3H_2O_{(l)} rightarrow NH_3 + 3OH^-_{(aq)} label{2}]

When nitrogen forms with other compounds it primarily forms covalent bonds. These are normally done with other metals and look like: MN, M3N, and M4N. These compounds are typically hard, inert, and have high melting points because nitrogen's ability to form triple covalent bonds.

Ammonium Ions
Nitrogen goes through fixation by reaction with hydrogen gas over a catalyst. This process is used to produce ammonia. As mentioned earlier, this process allows us to use nitrogen as a fertilizer because it breaks down the strong triple bond held by N2. The famous Haber-Bosch process for synthesis of ammonia looks like this:
[N_2 + 3H_2 rightarrow 2NH_3 label{3}]
Ammonia is a base and is also used in typical acid-base reactions.
[2NH_{3(aq)} + H_2SO_4 rightarrow (NH_4)_2SO_{4(aq)} label{4}]
Nitride ions are very strong bases, especially in aqueous solutions.
Oxides of Nitrogen
Nitrides use a variety of different oxidation numbers from +1 to +5 to for oxide compounds. Almost all the oxides that form are gasses, and exist at 25 degrees Celsius. Oxides of nitrogen are acidic and easily attach protons.
[N_2O_5 + H_2O rightarrow 2HNO_{3 (aq)} label{5}]
The oxides play a large role in living organisms. They can be useful, yet dangerous.
- Dinitrogen monoxide (N2O) is a anesthetic used at the dentist as a laughing gas.
- Nitrogen dioxide (NO2) is harmful. It binds to hemoglobin molecules not allowing the molecule to release oxygen throughout the body. It is released from cars and is very harmful.
- Nitrate (NO3-) is a polyatomic ion.
- The more unstable nitrogen oxides allow for space travel.
Hydrides
Hydrides of nitrogen include ammonia (NH3) and hyrdrazine (N2H4).
- In aqueous solution, ammonia forms the ammonium ion which we described above and it has special amphiprotic properties.
- Hyrdrazine is commonly used as rocket fuel
Applications of Nitrogen
- Nitrogen provides a blanketing for our atmosphere for the production of chemicals and electronic compartments.
- Nitrogen is used as fertilizer in agriculture to promote growth.
- Pressurized gas for oil.
- Refrigerant (such as freezing food fast)
- Explosives.
- Metals treatment/protectant via exposure to nitrogen instead of oxygen
References
- Petrucci, Ralph H, William Harwood, and F. Herring. General Chemistry: Principles and Modern Applications. 8th Ed. New Jersey: Pearson Education Inc, 2001.
- Sadava, David et al. LIFE: The Science of Biology. Eighth Edition. Sinauer Associate.
- Thomas, Jacob. Nitrogen and its Applications to Modern Future. San Diego State University Press: 2007.
Problems
- Complete and balance the following equations
N2+ ___H2→ ___NH_
H2N2O2 → ?
2NH3 + CO2 → ?
__Mg + N2 → Mg_N_
N2H5 + H2O → ?
- What are the different isotopes of Nitrogen?
- List the oxiadation states of various nitrogen oxides: N2O, NO, N2O3, N2O4, N2O5
- List the different elements that Nitrogen will react with to make it basic or acidic....
- Uses of nitrogen
Answers
- Complete and balance the following equations
N2+ 3H2→ 2NH3(Haber process)
H2N2O2 → HNO
Atomic No Of Nitrogen Dioxide
2NH3 + CO2 → (NH2)2CO + H2O
2Mg + 3N2 → Mg3N2
N2H5 + H2O → N2+ H+ + H2O
- What are the different isotopes of Nitrogen?
Stable forms include nitrogen-14 and nitrogen-15
Atomic No Of Nitrogen Periodic Table
- List the oxidation states of various nitrogen oxides: +1, +2, +3, +4, +5 respectively
Nitrogen Atomic Model
- List the different elements that Nitrogen will react with to make it basic or acidic :Nitride ion is a strong base when reacted with water, Ammonia is generally a weak acid
- Uses of nitrogen include anesthetic, Refrigerant, metal protector
